Osteoblasts - How It Is Affected By Biomaterial Implant Wear Debris
Total joint arthroplasty (TJA) is a type of surgery that has been used widely and successfully to treat joint problems caused by severe trauma or arthritis. It improves life quality, relieves pain, and restores joint function. But as time goes on, the long-term survival rate of implants goes down, which could lead to implant failure and the need for a second surgery.
Author:Suleman ShahReviewer:Han JuAug 07, 202232 Shares462 Views
Total joint arthroplasty(TJA) is a type of surgery that has been used widely and successfully to treat joint problems caused by severe trauma or arthritis.
It improves lifequality, relieves pain, and restores joint function. But as time goes on, the long-term survival rate of implants goes down, which could lead to implant failure and the need for a second surgery.
Implant failure is caused by many things, such as the way the material is made, how the parts are designed, how the surgery is done, biomechanical factors, the body's reaction, periprosthetic fracture, infection, and the implant coming loose.
The main reason for revision of arthroplasty failure is aseptic loosening following periprosthetic osteolysis.
Even though a lot has been written about how macrophages respond to wear debris, the balance of bone remodeling is also controlled by how osteoblastsmake new bone and how osteoclasts break down old bone.
According to mounting evidence, wear debris dramatically reduces osteoblastic physiology and subsequent bone production.
This article talks about how osteoblasts, osteoclasts, and macrophages work together to deal with wear debris. It also talks about possible ways to treat osteoblasts.
What Are Osteoblasts?
Preosteoblasts, immature osteoblasts, developing osteoblasts, and mature, matrix-producing osteoblasts make up the heterogeneous population of osteoblasts, which arise from pluripotent mesenchymal stem cells.
As the primary bone-forming cells, they produce a significant amount of type I collagen, which makes up 90% of the organic bone matrix, along with alkaline phosphatase, osteocalcin, and other components of the extracellular matrix.
By secreting matrix vesicles, osteoblasts also control the mineralization of the extracellular matrix.
Additionally, osteoblasts control osteoclasts by secreting osteoprotegerin (OPG) and receptor activator of nuclear factor (NF)-κB ligand (RANKL). RUNX2 (runt-related transcription factor 2), WNT, and BMP signaling pathways all regulate osteoblast differentiation.
After the matrix is made, osteoblasts can change into osteocytes that are embedded in the mineralized bone matrix, go through apoptosis, or turn into bone lining cells that are still and don't do anything.
While osteoblasts are important for bone production, they can also directly contribute to bone degeneration by secreting specific proteinases and preosteolytic mediators, altering cell viability, and modulating the expression of particular chemokines.
The capacity of osteoblasts to internalize wear particles, to exhibit cellular dysfunction, and to participate in wear particle-induced osteolysis is particularly significant in the context of this review article. During osteolysis, osteoblasts work with macrophages and osteoclasts. However, osteoblasts may not be as important as macrophages.

Osteoblasts and Osteoclasts
Osteoblastic Response To Orthopedic Wear Debris
Interaction Between Osteoblasts And Wear Debris
Osteoblasts engage in phagocytic and non-phagocytic interactions with particles. The response of macrophages to wear particles and the ensuing inflammatory response have been linked to oll-like receptor (TLR) signaling.
TLR involvement in the interaction between osteoblasts and particles hasn't been thoroughly elucidated, though. Even though osteoblasts aren't usually thought of as phagocytic cells, it has been seen that they can eat and absorb small pieces of debris into their cytoplasm.
When an effective phagocytosis inhibitor called cytochalasin D was used as a pretreatment, it stopped a lot of particles from getting inside the cells and stopped particle-induced changes in function like cell survival, differentiation, and inflammatory response. This suggests that phagocytosis is needed for particles to interact with osteoblasts.
Osteoblasts also take in wear debris through other channels. In fact, it has been observed that osteoblasts absorb particles through clathrin, caveolin, and micropinocytosis-mediated endocytosis.
However, relatively little research has been published on this subject so far. Even if it's to a smaller degree, particles may be able to interact with cells without being taken in by them.
Since cytochalasin D only partially stops the cell's response, it is interesting to think that phagocytosis-sized particles that don't get taken in by the phagocytosis pathway could still interact with osteoblasts.
Intracellular Organelle Damage
In addition, organelles including the vast Golgi complex, transfer vesicles, secretory granules, and electron-dense mitochondria that are required for the production of extracellular matrix proteins are abundant in the cytoplasm of osteoblasts.
After taking in wear debris, actin fibers, cell membranes, mitochondria, endoplasmic reticulum, and Golgi bodies show changes in their ultrastructure.
Actin staining indicated disordered actin filaments and extremely marked particle adhesion on actin fibers.
Additionally, osteoblasts displayed enormous Golgi extensions, less rough endoplasmic reticula, bigger, swollen mitochondria, and lysosomal-like components, which were all related to swelling of intracellular organelles brought on by disruption of the cell membrane.
Although no particles were discovered in the nucleus, further signs of DNA damage and DNA repair activation included supranuclear vacuolization, cell cycle halt in the G2/M phase, and an increase in the number of DNA fragments. If the particles have parts inside that look like lysosomes, this could mean that the particles kill cells directly.
Cell Cytoskeleton Disruption
Additionally, actin fibers are altered in osteoblasts. Significantly disturbed cytoskeletal architecture was seen by cytoskeleton staining, which was confirmed by strong particle attachment to actin fibers and jumbled actin filaments. Scientists have found a link between how flexible a cell is and how it senses changes in its environment.
As a result, even wear particles that do not cause cytotoxicity may nonetheless have a negative impact on long-term cell physiology due to modifications to the cytoskeletal organization caused by a subsequent event cascade.
Although wear particle-challenged osteoblasts have been shown to undergo cytoskeletal rearrangement modifications, little research has looked into how these changes affect cytoskeleton-dependent cell functions, including osteoblastic mechanosensing and cell differentiation.
Together, problems with the cytoskeleton may slow down the way cells work, but the exact mechanisms and pathways are still being studied.

Osteoblasts vs Osteoclasts | HOW DO THEY BOTH FUNCTION? Bone Remodeling
Alteration In Viability, Proliferation, Adhesion, And Migration
The viability, proliferation, adhesion, and migration of osteoblasts as well as other osteoblastic functions have all been widely shown to be adversely affected by various types of wear particles in a way that is dependent on the particle composition, size, dose, and time.
For future research, sub-cytotoxic levels were chosen as particle concentrations (like 0.1 mg/mL for Ti and 0.5 mg/mL for UHMWPE) that did not hurt cell viability or growth in tests.
Numerous studies have shown that this response changes depending on the particle material composition.
Particulate debris made of alumina and polystyrene is less dangerous than that made of metals and other materials. After 48 hours, Dalal et al.'s MG-63 cells were more sensitive to cytotoxicity from CoCrMo particles than from Ti, Zr-Oxide, and Zr alloy particles. At a concentration of 1 μm, CoCrMo particles stopped cell growth and viability the most.
In agreement with these results, Lenz et al. reported that Ti and ZrO2 had no effect, but CoCrMo particles more severely suppressed vitality. It is noteworthy that particulate CoCr seems to be less dangerous than any of its main metal components. The adhesion and migration of osteoblastic cells are similarly hindered by particle exposure.
The preservation of a stable cellular shape as well as cell adhesion and motility are made possible by the cell cytoskeleton and the assembly of actin filaments.
The loss of a significant number of related cellular processes is anticipated to be caused by the disassembly of cytoskeletal structures as a result of particle exposure. It has been shown that different particles reduce the spreading and adhesion strength of osteoblasts.
Osteogenic Differentiation
On exposure to wear particles, osteoblasts' ability to differentiate into osteoblasts and mineralize is also compromised.
These effects are demonstrated by the decreased expression of genes linked with early bone formation (such as ALP, Runx 2, and Osteoix) and late osteogenic markers (such as Osteocalcin), as well as the loss of their ability to synthesize type I collagen and regulate mineralization.
Even though some of these changes were not statistically significant, comparable trends were seen in the expression of transcription factors involved in osteoblastogenesis such as osteopontin (OPN), TGF-β1, sp7, bgalp, Fra-2 (fos related antigen-2), Dlx5, and Dkk1 (dickkopf-related protein 1).
In parallel to this, it has been discovered that osteogenesis temporarily up-regulates the expression of inhibitors of osteogenesis like BMP3, sclerostin (SOST), and Msx2.
Given that both BMP and WNT signaling were inhibited in osteoprogenitor cells, the particle-induced suppression of osteogenic differentiation is primarily mediated through Wnt/β-catenin and BMP/smad signaling pathways. Therefore, a potential therapeutic target could be one of these two signaling pathways. We'll talk more about this topic later.
Extracellular Matrix Imbalance
Osteoblasts actively participate in peri-implant matrix remodeling through the release of extracellular matrix and effectors like the matrix degradative proteinases MMPs (metalloproteinases) and their inhibitors TIMPs (tissue inhibitors of metalloproteinases).
However, periprosthetic loosening will be aided by pathological periprosthetic matrix remodeling. The majority of the extracellular matrix (osteoid) is made up of type I collagen.
In osteoblasts, wear particles have been shown to decrease the formation of type I collagen to varying degrees. The size, dosage, and composition of the particles all have an inhibiting effect.
According to Lochner et al., particles from Co and Cr strongly blocked the release of procollagen type I at a dose of 0.1 mg/mL. This inhibitory action requires a tyrosine phosphorylation cascade targeting the NF-κB signaling pathway.
Fibroblasts And Wear Particles
Seventy percent of the cells in the pseudosynovial membrane are fibroblasts. However, compared to macrophages, which make up just 15% of the cells in this membrane, they have garnered far less attention in investigations into the pathophysiology of wear-debris-associated osteolysis.
Numerous investigations have shown that fibroblasts participate in wear particle-induced osteolysis.
Wear particles have been demonstrated to promote fibroblastic RANKL expression through PGE2 receptor EP4 signaling, TLR-MyD88-RANKL, and the ER stress pathway both in vitro and in vivo, in addition to studies concentrating on fibroblast cytotoxicity and inflammation. It's interesting to see that autophagy seems to play a complex role in how wear particles and fibroblasts work together.
According to Wang et al., nano-sized Al2O3 wear particles encourage fibroblastic autophagy, which both in vitro and in vivo negatively influences the expression of RANKL and osteolysis. Poor autophagy in fibroblasts, on the other hand, made monocyte recruitment (C-X3-C motif chemokine ligand 1) better by making more CX3CL1 come out.
The Interactions Between Osteoblasts And Other Key Cells In Aseptic Loosening
In the peri-implant area, osteoblasts interact closely with osteoclasts, macrophages, and synovial membrane fibroblasts. These important cell types will inevitably interact given their close physical proximity. A conditioned medium has been utilized in in vitro techniques to examine this in greater depth.
Additionally, co-culture systems with various cell types have been used because they more nearly resemble the in vivo environment. To understand the issue of implant loosening as a whole, it is important to know how different cell types interact when wear particles cause disease.
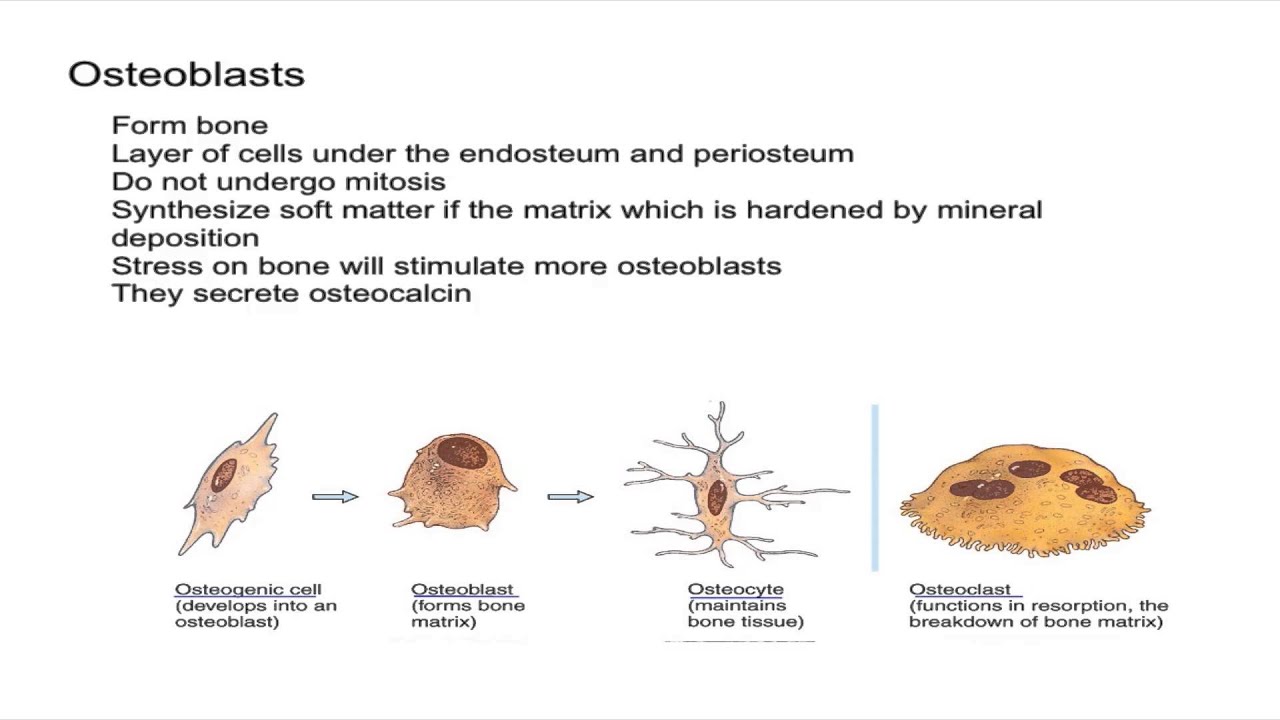
Osteogenic Cells and Osteoblasts
Limitations And Future Directions
For the therapyof aseptic loosening, osteoblasts may be a suitable therapeutic target. In fact, certain medications have shown promise in in vitro cell models of osteoblasts (MG-63, SaOS-2, and U-2 OS). In contrast to primary osteoblasts, osteosarcoma cell lines only show one stage of the development and phenotype of osteoblasts.
The response of osteoblasts to particles thus varies with the particular cell lines employed, and it appears that the activity of osteoblast-like cells under particle stimulation only partially mimics that of primary hOBs.
According to scanning electron microscopy, the morphology of the particulate materials created by implant wear varies more significantly than that of commercially produced particles, and they are more harmful or inflammatory to osteoblasts.
This supports the idea of investigating particle-cell interaction utilizing main hOBs and particles extracted from loosening implants. In addition, osteoblasts react differently to different kinds of wear debris according to the cell culture protocols, techniques, reagents, and biomaterials they are exposed to.
Additional research is necessary in order to explain these contradictions. For example, if different types of particles have different effects on peri-implant cells, figuring out why these differences happen could lead to material-specific treatments for periprosthetic osteolysis.
People Also Ask
What Is The Function Of The Osteoblasts?
As part of the skeletal development and remodeling process, osteoblasts are responsible for laying down new bone. Osteoblasts interact with osteocytes and haematopoietic stem cells directly during this process.
Do Osteoblasts Break Down Bones?
Both the modeling and remodeling of bone require cells known as osteoblasts, which are responsible for the formation of bone, and osteoclasts, which are responsible for the breakdown of bone.
What Is An Example Of Osteoblasts?
The mandibular and clavicular bones were created in this manner, as was the skull. When osteoblasts move to connective tissue membranes, they deposit a bone matrix around the osteocytes.
Where Are Osteoblasts Found In Bone?
Osteoblasts are cuboidal cells that are found along the bone surface and make up between 4 and 6% of the total resident bone cells. Osteoblasts are best known for making bone, which is what most of them do.
Conclusion
Aseptic loosening is significantly influenced by osteoblasts, which are important players in the creation of bone structure. In vitro models show that when osteoblasts are exposed to wear particles, many cellular processes are turned off, proinflammatory mediators are released, and different signaling pathways are either turned on or turned off.
These responses both directly and indirectly contribute to osteoclastic bone resorption and osteoblastic bone creation. Furthermore, powerful pharmacological treatments that target osteoblasts in vivo are being developed on the basis of these in vitro models. More research is required to clarify the precise roles of osteoblasts in aseptic loosening and to prove their potential value as a therapeutic target.

Suleman Shah
Author
Suleman Shah is a researcher and freelance writer. As a researcher, he has worked with MNS University of Agriculture, Multan (Pakistan) and Texas A & M University (USA). He regularly writes science articles and blogs for science news website immersse.com and open access publishers OA Publishing London and Scientific Times. He loves to keep himself updated on scientific developments and convert these developments into everyday language to update the readers about the developments in the scientific era. His primary research focus is Plant sciences, and he contributed to this field by publishing his research in scientific journals and presenting his work at many Conferences.
Shah graduated from the University of Agriculture Faisalabad (Pakistan) and started his professional carrier with Jaffer Agro Services and later with the Agriculture Department of the Government of Pakistan. His research interest compelled and attracted him to proceed with his carrier in Plant sciences research. So, he started his Ph.D. in Soil Science at MNS University of Agriculture Multan (Pakistan). Later, he started working as a visiting scholar with Texas A&M University (USA).
Shah’s experience with big Open Excess publishers like Springers, Frontiers, MDPI, etc., testified to his belief in Open Access as a barrier-removing mechanism between researchers and the readers of their research. Shah believes that Open Access is revolutionizing the publication process and benefitting research in all fields.

Han Ju
Reviewer
Hello! I'm Han Ju, the heart behind World Wide Journals. My life is a unique tapestry woven from the threads of news, spirituality, and science, enriched by melodies from my guitar. Raised amidst tales of the ancient and the arcane, I developed a keen eye for the stories that truly matter. Through my work, I seek to bridge the seen with the unseen, marrying the rigor of science with the depth of spirituality.
Each article at World Wide Journals is a piece of this ongoing quest, blending analysis with personal reflection. Whether exploring quantum frontiers or strumming chords under the stars, my aim is to inspire and provoke thought, inviting you into a world where every discovery is a note in the grand symphony of existence.
Welcome aboard this journey of insight and exploration, where curiosity leads and music guides.
Latest Articles
Popular Articles